Here in Vermont, during the warm days of late August through the frosts of October, children (and adults too) love to find wooly bear caterpillars (Grammia incorrupta, Pyrrharctia isabella and also Platyprepia virginalis) crawling through the dooryard, or crossing a dirt road or trail. They are cute: fuzzy, some banded in brown and black, they are easy to recognize. Old farmers will tell you that the thickness of the bands relate to the length and severity of the coming winter: there can be a lot of variation in banding patterns, and the caterpillars do seem different from season to season. Some of this variation may be genetic (sort of like hair color for humans), but some may be a variation in what biologists call phenotype (the pattern, or collection of patterns, of gene expression). Variations in phenotype occur in all life forms: for example, consider twin studies where individuals with identical genomes are separated at birth and raised in different environments. Often, these individuals express different phenotypes: while their hair color might be the same, their body shape, patterns of illness, personality and even size might be quite different depending on circumstances.1 So it may be possible that different environmental conditions during spring and summer, when the wooly bears are maturing, could lead to different phenotypes the following year.

[FIGURE 1: Pyrrharctia isabella, the wooly bear caterpillar]
As far as I know, we lack objective data on phenotypic variation in wooly bear banding patterns and if that variation correlates to future weather (and this dearth of data might be a good thing, because otherwise we might not be able to enjoy spirited debates centered on caterpillar-based snowfall predictions). But there is another aspect of a wooly bear’s phenotype that researchers like Richard Karban from the University of California, Davis have been able to observe objectively: the changes in browsing behavior that occur when a caterpillar is infected by the minuscule tachinid fly (Thelaira americana).2 These common parasites cause life-threatening illness: in lab conditions, only 30-40% of caterpillars survive and reproduce when infected compared to a normal rate of about 80%. Wooly bears normally browse on the common flora found wild in fields: plants like lupins (one of their favorites). When they are infected, however, they seek out different plants: in some cases, it’s poison hemlock (Conium maculatum, source of the highly toxic alkaloid coniine);3 in others, it’s botanicals like borage (Borago officinalis) which contain mildly toxic pyrrolozidine alkaloids;4 in yet other cases, caterpillars will choose plants rich in bitter iridoid glycosides (like Plantago lanceolata).5 If healthy wooly bears are fed these plants exclusively, fewer individuals survive to maturity: only about 60-70%. These plants are somewhat toxic to the caterpillars themselves. So why, if given a choice, would they consume them at all?
As it turns out, poison hemlock and species rich in pyrrolizidine alkaloids are toxic to tachinid flies. This toxicity makes it so that if infected caterpillars consume these plants, more survive: close to 60% reach maturity.6

[FIGURE 2: The characteristic spotted stem of Conium maculatum]
This observation is remarkable in and of itself: these wooly bears are behaving like herbalists, seeking out medicinal species to treat their infection. Their treatment protocol seems effective too, since it almost doubles survival. They are using plants that are somewhat toxic to them, and making a “calculated” decision to deal with this toxicity instead of dealing with the infection unassisted: this is the same calculus we humans use for medical interventions. So how does this happen? Wooly bears lack an oral tradition and mythology, there are no caterpillar elders (outside of Wonderland) who can explain to the new generation how to treat tachinid infection. Seeking out these medicinal plants seems to be an innate part of their browsing behavior – meaning that, in their genome, there are instructions on how to distinguish and feed on a range of different plants in a range of different conditions, including tachinid infection. If we think of behavior patterns as part of the caterpillars’ phenotypes, then when they are infected with tachinids, wooly bears exhibit different phenotypes. If we could take two twin wooly bears, separate them at birth, and infect only one of them with the parasitic flies, we would observe these two different phenotypes: one clover-browsing, and one borage-browsing. This variation opens up even more interesting possibilities, as we shall see.
While insect self-medication is a relatively new discovery,7 we’ve known for a long time that primates (humans included) use plants medicinally.8 Chimpanzees, bonobos and gorillas use plants to prevent infection: certain species of fig (such as Ficus glabrata) contain useful enzymes that, when consumed regularly, lessen the chance of parasitic worm infection. Michael A. Huffman, a biologist at the Primate Research Institute at the University of Kyoto, Japan, has documented and detailed these behaviors and more: sometimes, primates will seek out the root of iboga (Tabernanthe iboga, a hallucinogenic plant),9 consume it, and run around the forest erratically for a bit. They will also use the bitter inner pith of Vernonia species to clear nodular worm infections: based on Huffman’s observations, this treatment is quite effective, often expelling the worms in less than 24 hours.10
Remarkably, primates use these herbs methodically, with many of the same techniques we employ when working with medicinal plants.
For example, in the case of Vernonia, chimpanzees will often travel long distances to find the relatively rare plant (they’re not just looking for random inner pith from any old species – they have wildcrafting skills); they peel off and discard the leaves and outer bark, isolating the important part (they have pharmacy skills); non-infected individuals don’t touch Vernonia, even if it’s present in their community, and the use of the plant peaks only in the wet season when nodular worm infections are more common (they have diagnostic and prescribing skills). Specific medication for specific conditions, coupled with wildcrafting and medicine-making skills: this suite of behaviors is almost identical to ours.

[FIGURE 3: Vernonia amygdalina]
Huffman summarizes his findings this way: the behavior patterns of animals change in response to changes in their internal or external environments; some baseline behaviors, like fig consumption, grant access to medicinal phytochemistry that helps prevent infection (in primates, about 15-20% of plants consumed fit this description); and finally, specific self-medication behaviors, which exhibit sophisticated diagnostic and pharmacy skills, happen in response to specific conditions.11 While his research focuses on primates, all of the above also seems to apply to wooly bears too: herbalism is part of being an animal, from insects to us. So what makes a gorilla’s herbalism different from a wooly bear’s?
Researchers agree that a caterpillar’s herbalism is innate. A primate’s herbalism, however, includes cultural elements: older adults will show younger members of the community how to find important medicinal species and how to prepare them.12 For many years, this is how we thought humans learned plant medicine: by observing and mimicking animal culture. The trial-and-error method of learning herbalism seems impossibly complicated and fraught with danger, so perhaps observing animals gave us a “shortcut” to the more useful species (so the argument goes). But the wooly bears give us a different perspective: there is no cultural encoding of knowledge in their case. So if insects lack culture, if their herbalism is an innate birthright, how is it passed on? Why is the self-medication behavior that caterpillars exhibit visible only under specific circumstances (i.e. tachinid infection)? The shift in browsing behavior is very specific, and won’t occur in the absence of infection: the innate knowledge isn’t just about which plants to use, but when to use them. It turns out that this behavioral variation is encoded in the genome, and driven by evolution: this is a concept known as phenotypic plasticity, or the capacity to embody different phenotypes in different situations.13
Having this plasticity, this flexibility to alter behavior (or other characteristics, like size and shape), seems like it might increase fitness and survival: after all, when the environment changes, having some options might make you more likely to find a way to cope. But it turns out that for plasticity to be adaptive (able to increase your fitness for survival and reproduction), it needs to offer options that mirror the options that are available in the novel environment.14 If, for example, wooly bears were exposed to a new infection that the species had never encountered (i.e. not tachinid flies), and in response they shifted their behavior to eating more toxic plants, but the new infective organism was unaffected, then their phenotypic plasticity could actually be counter-adaptive. Fewer of the wooly bears who eat the toxic plants would survive compared to individuals who lacked that plastic response (it is important to remember that there are different degrees of phenotypic plasticity in any given population). Over time, the exposure to the novel infection would begin to remove that plastic response, narrowing the range of browsable plants, and discarding the self-medicating behavior (a process known as genetic “canalization”).15 So phenotypic plasticity only becomes adaptive when the environment is different, but retains enough of the cues that were present in an organism’s historical range.
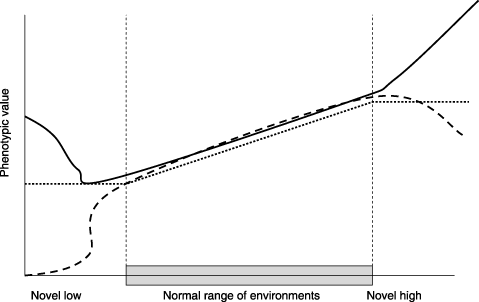
[FIGURE 4: Phenotypic response in different environmental ranges. Reprinted from Ghalambor 2007]
When the novel environment is completely different from an organism’s historical range, all bets are off. In familiar environments, there may have been a linear plastic response: as tachinid fly infection increased, toxic plant browsing increased, and vice versa. But in wholly novel situations, we can’t be sure: different responses, not historically subject to the constraints of evolution, will now be exposed leading to wildly unpredictable phenotypes.16 In the end, this is an adaptive strategy, too: individuals with useful responses will have more offspring, and those traits will be retained. This is a pattern that happens all the time in living systems: take, for example, our sensitivity to the hormone leptin, geared to suppress appetite during times of abundance. Historically, individual humans have more or less body fat depending on the availability of food resources, which fluctuate seasonally. As we eat more and lay down more body fat, leptin levels rise, suppressing feeding behavior. During drier seasons, we use up our fat reserves and leptin levels drop, increasing hunger and the drive to find food. This seems to work fairly well, and predictably for all humans, in normal body-fat ranges – but in some individuals, when body fat levels rise to historically unprecedented levels, a leptin “resistance” develops where leptin stops working. This isn’t true for all humans, but for some folks it is.17 The point is that the predictable relationship between body fat and leptin breaks down when the organism leaves the historical body fat and leptin ranges: some folks retain leptin sensitivity, others exhibit resistance. Over time, the individuals who retain sensitivity might have greater fitness – and this will refine the plastic response of the entire population making us (hopefully) more tolerant to higher body fat ranges.
So we have phenotypic plasticity, which works well in historical environmental contexts, and we have adaptive phenotypic plasticity, which can lead to evolutionary changes in a population that is attempting to colonize a new environment – so long as that new environment isn’t completely different from the historical context (in these latter cases, extinction can be a real threat). To use our example of wooly bears, imagine a novel environment with an infection that was sensitive to Conium but not to pyrrolizidine alkaloids. While all wooly bears modify their behavior when infected, those who tend to browse more on Conium will do a lot better in this new environment than those who browse on pyrrolizidine-rich plants. Who knows, some wooly bears may have genes that lead them to a third type of plant which, historically, didn’t help but which might be useful now. Over a few generations, the wooly bears will begin to modify their plasticity: the behavior-modifying genes are still present, and allow them to colonize this novel environment, but the specific phenotypic options available will start to change, leaving behind pyrrolizidine-browsing behavior and adopting a connection to the new plants. Crucially, having the ability to browse on Conium gives the wooly bears a hedge against extinction: the plasticity is adaptive, and helps the population colonize its new niche with its new challenges.
What can we learn from the understanding that populations display phenotypic plasticity? How does this affect adaptation and resilience in changing times and in novel environments? What might all this mean for us, and for the long-term relationships we’ve had with plants, mushrooms, and bacteria during the course of our evolutionary history?
First off, it is important to remember that the use of medicinal plants in deliberate, effective ways has been part of the range of possible phenotypic responses of animals since the very beginning. That is to say, using herbs to increase survival (both as tonics and as therapeutic substances) is built right in to living beings as part of a range of possible responses to shifts in their environment. Wooly bears know it, and so do we. This may seem like a simple conclusion, but it also is not necessarily one that our culture has embraced as part of its worldview. So I will repeat it again, since it should be emphasized:
even the simplest life-forms deliberately shift their behavior when they’re sick, and part of that shift involves using medicinal plants that aren’t used in times of health.
Basic wildcrafting, pharmacy, and herbal medicine are as much our birthright as the wooly bear’s.
Furthermore, since phenotypic plasticity relies on information stored in our genomes, our innate herbalism is an encoding of our ancestors’ collective experience: those who came before retained plasticity sufficient to behave differently in a range of different environmental conditions, and the spectrum, or range, of reactions (which include herbalism) were retained and passed on to their offspring.18 The fact that wooly bears browse on toxic plants when parasitized by tachinid flies is a reflection that, historically, wooly bears’ environments included many different kinds of plants and tachinid flies, too. The caterpillars that retained a plasticity of browsing behaviors (including herbalism) survived in greater numbers and passed on their genes. Our genes don’t simply encode a single road: they contain instructions for different patterns of behavior keyed to different environmental conditions. These behaviors, and the environmental conditions that elicit them, are our ancestors’ stories.
Another interesting point is that this behavioral/phenotypic plasticity can be lost.19 Without the correct environmental cues (or resources), any advantage conferred by the plasticity is diminished, and those genes can become too costly to maintain. When wooly bears can’t access toxic plants because there are none in their environments, individuals who waste energy looking for them won’t do as well. Over time, the self-medicating drive of the population will be lost: maintaining the full range of plastic response in the face of infection isn’t worth it if the medicine you’re looking for isn’t around. Canalization, the pruning away of now-irrelevant ancestral experience, will ensue. So engaging with the medicinal plants our ancestors used isn’t just an occasionally-helpful behavior: if we don’t stay engaged, we run the risk of losing herbalism as a resource.
The flip side of this is that our environment is shifting dramatically. There are new cues and signals, from plasticizers to dioxins, from glyphosate to pharmaceuticals. It would be hard to argue that (thanks mostly to us humans) we are moving into a new environmental range, one our ancestors may not have had a chance to experience. Our biosphere is attempting to colonize this novel environment and adapt to these changes. What we’ve seen from research into adaptive plasticity is that evolution occurs best when the novel environment is different from ancestral ones, but still retains many features of the historic range. Think about it: if the environment is identical to that of the past, little adaptation will occur because the range of plasticity is perfectly matched to the environmental range. If, on the other hand, the environment is so radically different that none of the cues or resources for past plasticity are present, then the population may likely die out (except, perhaps, for some lucky random mutants). For adaptation, we need to let a plastic response loose in an environment that is similar enough to historic ranges to allow old tools to work, while also providing enough novelty to reshape the range of phenotypic plasticity. This is how we evolve.

[FIGURE 5. Herbal gardens can be important sources of biodiversity]
There are important implications here. One way or another, like it or not, we are journeying into the future. Along this journey, we are seeing novel challenges: things like insulin and leptin resistance, and perhaps also the increase in certain types of cancer, are in part evidence that we aren’t perfectly matched to the novel environments the future might hold. This is an opportunity for evolution, but it will only be effective if we maintain our connection to the ancestral experiences encoded in our genes, which includes consuming a wide spectrum of plants and mushrooms, the same way we’ve always done. If we choose not to honor this encoded experience, we turn our backs on a genome-stabilizing force. Take this specific example: phytoestrogens, and specifically isoflavones (a class of polyphenolic molecule found mostly in the Fabaceae), alter our gene expression, moving us away from a pro-breast-cancer phenotype. Modern plasticizers and other xenoestrogens (there are so many) do the opposite: they elicit the breast cancer phenotype in a way we’ve never seen before.20 Over time, we might be able to adapt to ubiquitous xenoestrogens – but this becomes much less likely if we don’t have access to isoflavones which can help buffer the threats in our modern world.
So we come to herbalism. As we move into the future, it would seem that we stand a better chance of adapting successfully if we work to retain as many of the historically-relevant environmental cues we know. Unhybridized, wild, medicinal plants and mushrooms hold many of these cues in the form of molecules that interact at specific sites in our cells, across our physiologies. The can serve as an anchor for our future – but perhaps this is not the best analogy, as anchors tend to be a drag on forward momentum, and that is not what medicinal herbs are. These herbs, and the signals they provide, are more like parents: a supportive safety net, a context from which we can venture into new territory, grow, and adapt successfully. This context will allow us to maintain an effective range of responses as the world changes. Our genes encode this ancestral memory for us: the memory, as real today as it was when the first insects evolved, of how plant power keeps us strong. It is more than simply useful: it is essential to our long-term survival.
I leave you with this final thought: none of us are separate from our environments.
The herbs with which we co-evolved react along with us to environmental shifts. If we stay connected to them, not only will we adapt more effectively, but our patterns of behavior will change, too: as we repeatedly see, herbs can support us through so many of the challenges of our modern world. And it is my belief, though I take it a bit on faith, that healthy, well-supported humans make more sustainable choices, choices that are more thoughtful and aware of context, choices that are less reactionary and less fear-based. This, in the end, can only benefit our entire biosphere. Herbalism is a technology that the ecology itself uses to build and stabilize its own wellness. We and the wooly bears are just pieces of this wellness ecology, part of the dance – if, that is, we can open our ears to the music.
1. Wong, Albert HC, Irving I. Gottesman, and Arturas Petronis. "Phenotypic differences in genetically identical organisms: the epigenetic perspective." Human molecular genetics 14.suppl_1 (2005): R11-R18. APA
2. Find Karban’s long-term research project overview and data here: https://karban.wordpress.com/ltreb/ Accessed 11/21/2017
3.Karban, Richard, and Gregory English-Loeb. "Tachinid parasitoids affect host plant choice by caterpillars to increase caterpillar survival." Ecology 78.2 (1997): 603-611.
4. Singer, Michael S., Kevi C. Mace, and Elizabeth A. Bernays. "Self-medication as adaptive plasticity: increased ingestion of plant toxins by parasitized caterpillars." Plos one 4.3 (2009): e4796.
5. Stamp, Nancy E., and M. Deane Bowers. "Foraging behaviour of caterpillars given a choice of plant genotypes in the presence of insect predators." Ecological entomology 25.4 (2000): 486-492.
6. English-Loeb, Gregory M., Alison K. Brody, and Richard Karban. "Host-plant-mediated interactions between a generalist folivore and its tachinid parasitoid." Journal of Animal Ecology (1993): 465-471.
7. For a good review, see Michael Singer’s work. Singer, Michael S. "Evolutionary ecology of polyphagy." Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects (2008): 29-42.
8. Huffman, Michael A. "Primate Self-Medication, Passive Prevention and Active Treatment-A Brief Review." International Journal of multidisciplinary Studies 3.2 (2016).
9. Cousins, Don, and Michael A. Huffman. "Medicinal properties in the diet of gorillas: an ethno-pharmacological evaluation." (2002).
10. Koshimizu, Koichi, Hajime Ohigashi, and Michael A. Huffman. "Use of Vernonia amygdalina by wild chimpanzee: possible roles of its bitter and related constituents." Physiology & behavior 56.6 (1994): 1209-1216.
11. Huffman, Michael A. "THE STUDY OF PRIMATE SELF-MEDICATION A collection of multidisciplinary research work by members of The CHIMPP Group*(the first 18 years, 1987–2005)." Primate research institute, Kyoto University, Kanrin p41-42, Inuyama. Back to cited text 40 (2005).
12. Huffman, Michael A., and Satoshi Hirata. "An experimental study of leaf swallowing in captive chimpanzees: insights into the origin of a self-medicative behavior and the role of social learning." Primates 45.2 (2004): 113-118.
13. West-Eberhard, Mary Jane. "Phenotypic plasticity and the origins of diversity." Annual review of Ecology and Systematics 20.1 (1989): 249-278.
14. For a good review of adaptive and non-adaptive phenotypic plasticity, examples, and how these factors affect the evolution of a population, see: Ghalambor, Cameron K., et al. "Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments." Functional ecology 21.3 (2007): 394-407.
15. West-Eberhard, Mary Jane. Developmental plasticity and evolution. Oxford University Press, 2003.
16. Rutherford, Suzanne L. "From genotype to phenotype: buffering mechanisms and the storage of genetic information." Bioessays 22.12 (2000): 1095-1105.
17. Myers, Martin G., Michael A. Cowley, and Heike Münzberg. "Mechanisms of leptin action and leptin resistance." Annu. Rev. Physiol. 70 (2008): 537-556.
18. AUBIN‐HORTH, N. A. D. I. A., and Susan CP Renn. "Genomic reaction norms: using integrative biology to understand molecular mechanisms of phenotypic plasticity." Molecular Ecology 18.18 (2009): 3763-3780.
19. Debat, Vincent, and Patrice David. "Mapping phenotypes: canalization, plasticity and developmental stability." Trends in Ecology & Evolution 16.10 (2001): 555-561.
20. There are many compounds with estrogenic activity. Most of them have different selectivity for different types of estrogen receptors. Depending on which receptors are affected, the compounds can stimulate cell growth and division, or suppress it. For a comprehensive overview see: Lorand, T., E. Vigh, and J. Garai. "Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: phytoestrogens and xenoestrogens." Current medicinal chemistry 17.30 (2010): 3542-3574.